Abstract
Background: Hemophilia A (HA) is an inherited blood disorder caused by deficiency or dysfunction of coagulation factor VIII (FVIII), for which a number of gene therapies are currently in clinical trials. SPK-8011 is a recombinant adeno-associated viral (AAV) vector composed of SPK200 (the bioengineered AAV capsid LK03) with a hepatotropic, truncated transthyretin enhancer and promoter, and F8 complementary DNA encoding a B-domain-deleted FVIII. The aim of SPK-8011 therapy is to provide long-term stable FVIII expression and prevention of bleeding. A Phase I/II trial of SPK-8011 has demonstrated multiyear sustained FVIII expression and a notable reduction in bleeding episodes, with no major safety concerns (George et al. New Engl J Med 2021).
AAV vectors are thought to pose minimal risk for vertical or horizontal transmission since they are replication incompetent. Assessment and characterization of vector shedding, a phenomenon by which viral vector is released into the environment via excreta of the treated participant (e.g. blood, feces, saliva, semen, tears, and urine), is an important part of clinical development. Precautions are currently recommended for the handling of these potentially vector-containing bodily fluids, including the exclusive use of barrier contraception until vector clearance. Here, we report data on vector clearance from bodily fluids from the Phase I/II trial of SPK-8011.
Methods: In this open-label, multicenter, non-randomized dose-escalation trial (NCT03003533/NCT03432520), males with moderate or severe HA were infused with SPK-8011 at one of four doses ranging from 5×1011 to 2×1012 vector genomes (vg) per kilogram of body weight. Participants had baseline FVIII activity of ≤2% of normal levels and were negative for neutralizing antibodies to SPK200.
A vector shedding assay based on the TaqMan probe quantitative polymerase chain reaction (qPCR) technique was developed, optimized, and validated by QPS Holdings LLC to quantify the SPK-8011 viral vector genome DNA in serum, peripheral blood mononuclear cells (PBMCs), saliva, semen, and urine (full methodology published in George et al. New Engl J Med 2021). Total DNA was extracted and quantitated from semen and PBMCs, and total nucleic acid was extracted from serum, saliva, and urine. The resulting DNA or eluate was analyzed by mass or volume, as applicable (≤1μg DNA or 5μL), for SPK-8011 concentration, in triplicate, by quantitative SPK-8011 qPCR assay. Vector shedding was estimated by quantitation of vector genome levels using a qPCR assay and so may capture DNA fragments. Test samples from screening/baseline and/or day 0 (prior to vector infusion) were analyzed for all participants. For each matrix, subsequent timepoints were analyzed until samples at three consecutive timepoints tested negative or had results below the quantification limit. The concentration of SPK-8011 was reported in vector genome (copies) of SPK-8011 per mL of sample processed, and per μg of DNA for PBMCs and semen.
Results: A total of 23 men (aged 18 to 52 years) have received SPK-8011 in this trial to date. The data cut-off for this analysis was 30 June 2022, by which time vector clearance results were available for 21 participants.
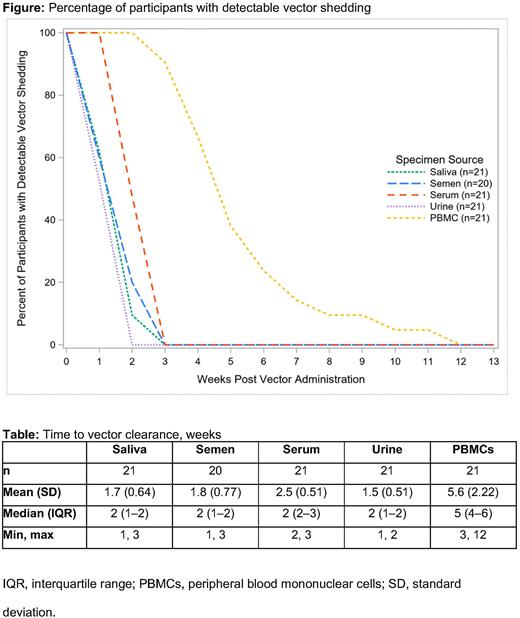
Across all dose cohorts, vector genome concentration was below the quantification limit for all participants by 3 weeks following vector infusion in saliva, semen, serum, and urine; and by 12 weeks following vector infusion in PBMCs (Figure). Median (interquartile range [IQR]) time to vector clearance in saliva, semen, serum, urine, and PBMCs was 2 (1-2) weeks, 2 (1-2) weeks, 2 (2-3) weeks, 2 (1-2) weeks, and 5 (4-6) weeks, respectively (Table). Median time to vector clearance was consistent across all vector doses administered.
The presence of vector DNA or nucleic acid in bodily fluids was not associated with any reported adverse events.
Conclusions: Following a single infusion of SPK-8011, time to clearance was fastest in urine, saliva, semen, and serum, with vector genome concentration below the quantification limit for these matrices in all participants by 3 weeks, and by 12 weeks in PBMCs. Knowing the rate of clearance in saliva, semen, and urine allows boundaries to be set for the duration of sample collection and safety precautions such as barrier contraception, thereby reducing the patient burden in the clinical trial setting.
Disclosures
Tran:Sanofi: Research Funding; AstraZeneca, CSL Behrig: Honoraria; Pfizer, Takeda: Speakers Bureau. Eyster:PennState Hershey Medical Center: Current Employment; SPARK/Roche, Novo Nordisk and Baxalta: Research Funding. Croteau:Spark Therapeutics, Genentech, Sanofi: Research Funding; Bayer, Pfizer: Honoraria; ATHN, Hemophilia Alliance, THSNA: Membership on an entity's Board of Directors or advisory committees; Bayer, Pfizer, Sanofi, BioMarin, HemaBiologics: Consultancy. Ragni:BioMarin: Honoraria; Takeda: Honoraria, Other: Provided study (rVWF) drug for trial. Samelson-Jones:Spark Therapeutics, Pfizer, Accugen, Cabaletta, Uniqure, Frontera: Research Funding. Sullivan:Octapharma: Consultancy; Biomarin: Consultancy, Speakers Bureau; Pfizer: Consultancy; Genentech: Consultancy; Bayer: Consultancy; Mississippi Center for Advanced Medicine: Current Employment; Make-A-Wish Mississippi: Membership on an entity's Board of Directors or advisory committees. Rasko:Gilead, Roche, Novartis, Bluebird Bio, SPARK therapeutics, Cynata, Pfizer Inc: Consultancy; Genea: Current equity holder in publicly-traded company; Rarecyte: Current equity holder in private company. Jaworski:Spark Therapeutics: Current Employment. MacDougall:Roche: Current equity holder in publicly-traded company; Spark Therapeutics: Current Employment. Jaeger:BioNTech: Ended employment in the past 24 months; Spark Therapeutics: Current Employment. Hofer:Spark Therapeutics: Current Employment; Merck, Novo Nordisk: Current equity holder in publicly-traded company; Roche: Current equity holder in private company. Li:Spark Therapeutics: Current Employment; MilliporeSigma: Ended employment in the past 24 months; Roche: Current equity holder in publicly-traded company. Mingozzi:American Society of Gene and Cell Therapy: Membership on an entity's Board of Directors or advisory committees; Roche pharma: Current equity holder in publicly-traded company; Spark Therapeutics: Current Employment. Chang:F. Hoffmann-La Roche Ltd: Current equity holder in publicly-traded company; Spark Therapeutics: Current Employment. Levy:Spark Therapeutics, Inc Board of Directors (Non-voting): Membership on an entity's Board of Directors or advisory committees; Roche Pharmaceuticals: Current equity holder in publicly-traded company; Spark Therapeutics Inc: Current Employment; Genentech, Inc: Ended employment in the past 24 months.
Author notes
Asterisk with author names denotes non-ASH members.